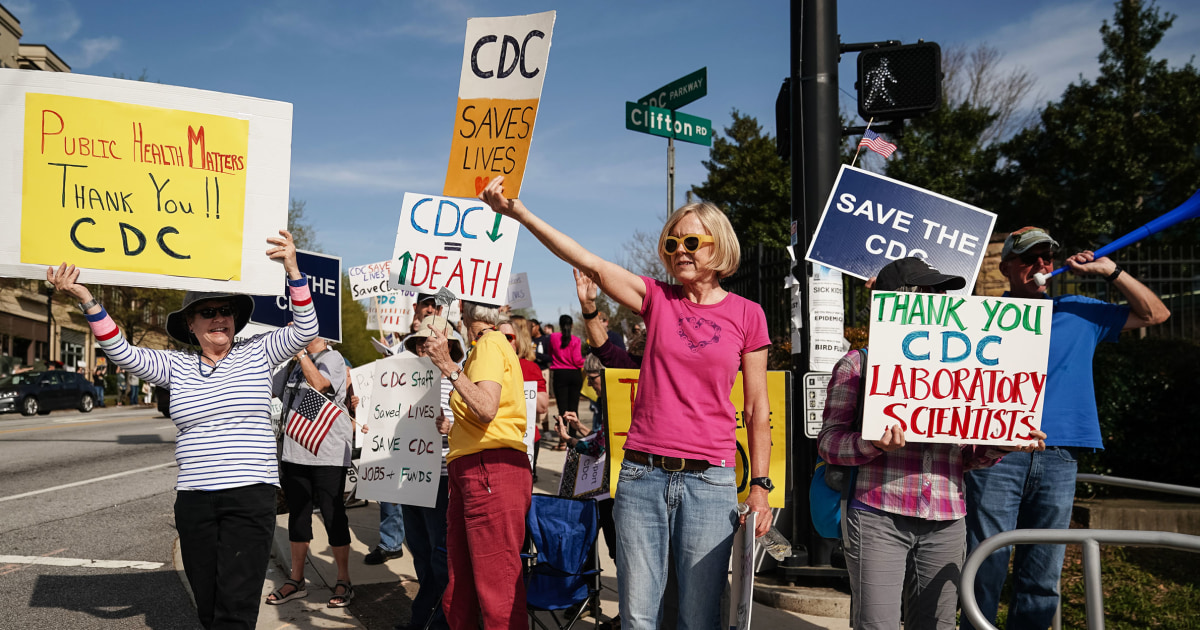CDC Vaccine Advisory Panel Changes and New Influenza Vaccine Recommendations
Health Secretary Robert F. Kennedy Jr. has formed a new vaccine panel, while federal recommendations for annual flu shots now exclude thimerosal as a preservative.
Overview
Health Secretary Robert F. Kennedy Jr. replaced the CDC's 17-member vaccine advisory panel with a seven-member group critical of vaccines.
The new panel will review immunization schedules for children, raising public health concerns about vaccine trust and safety.
A federal panel recommends that all Americans over six months old receive an annual influenza vaccine without preservatives like thimerosal.
Despite thimerosal's use in multi-dose vials, studies show no link to autism or neurological effects, addressing public concerns.
The CDC's ACIP committee typically determines vaccination recommendations, emphasizing the importance of the new panel's decisions on public health.
Analysis
Center-leaning sources frame the CDC's vaccine committee discussions around thimerosal as contentious, highlighting concerns from public health experts and pediatric organizations. Implicit bias suggests skepticism towards the committee's credibility, while emphasizing the scientific consensus against thimerosal's link to autism. The narrative reflects anxiety over potential vaccine hesitancy.
Sources (27)
Center (12)
FAQ
Health Secretary Robert F. Kennedy Jr. replaced the CDC's 17-member vaccine advisory panel with a seven-member group critical of vaccines to review immunization schedules and vaccine safety, reflecting a shift towards more vaccine skepticism within the advisory body.
The federal panel now recommends that all Americans over six months old receive an annual influenza vaccine that excludes thimerosal as a preservative, addressing public concerns despite studies showing no link to autism or neurological effects.
There are public health concerns that the new panel's review of immunization schedules, including cumulative vaccine effects on children and adolescents, could undermine vaccine trust and safety, especially as some members are vaccine skeptics.
The AAP quickly pushed back against plans to reconsider hepatitis B vaccination for newborns, stating that ignoring the success of vaccination programs or not vaccinating newborns could be unscientific and dangerous due to the risk of deadly virus transmission at birth.
History
This story does not have any previous versions.