US Vaccine Panels Shift COVID-19 Guidance to Individual Choice, Delay Newborn Hepatitis B Vote, and Restrict MMRV
US vaccine committees shift COVID-19 guidance to individual choice, postpone newborn hepatitis B decisions, and restrict MMRV for young children, reflecting a turbulent policy landscape.
Overview
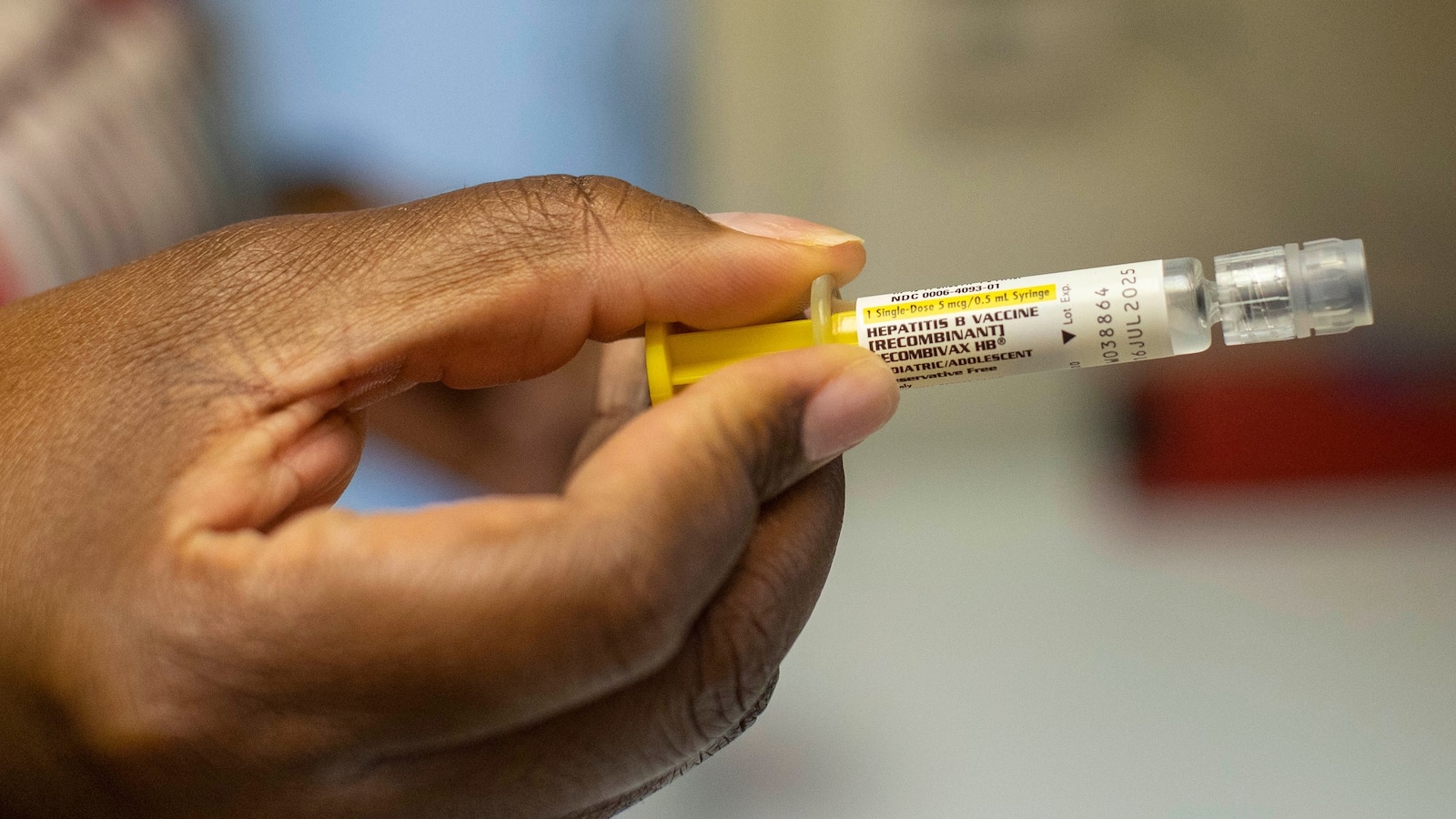
US vaccine advisory panels have postponed votes on delaying the first dose of the hepatitis B vaccine for newborns, particularly for those whose mothers test negative, due to ongoing debate and skepticism regarding its necessity at birth.
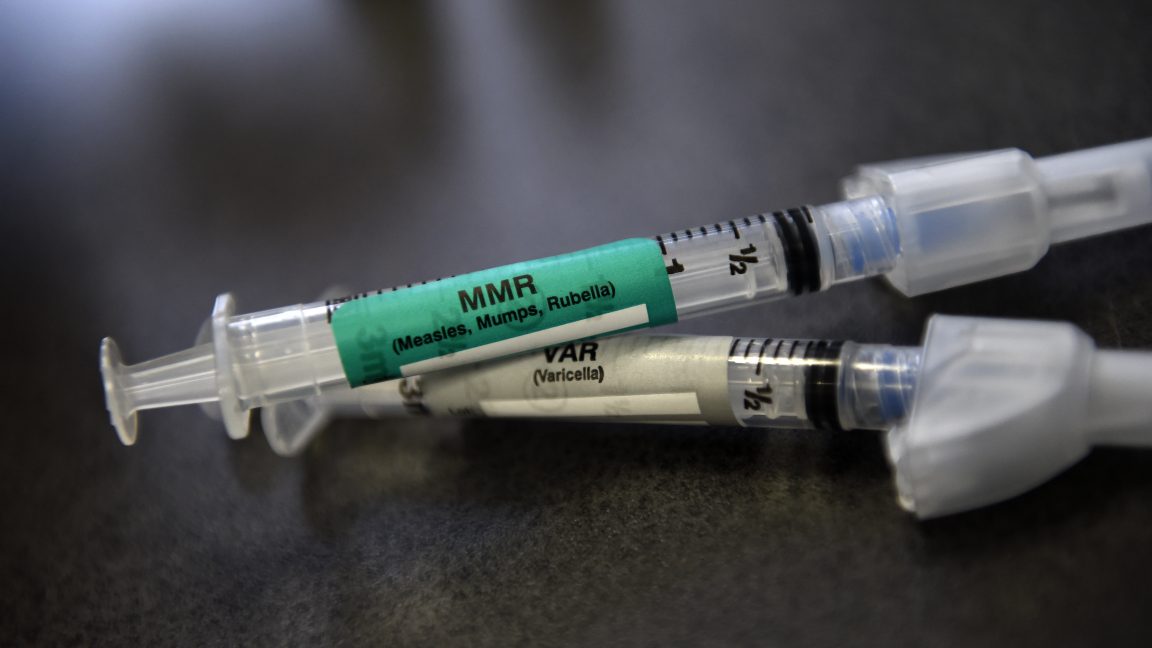
Committees have voted to stop recommending the combined MMRV vaccine for children under four, instead advising separate MMR and varicella shots due to a slight increased risk of febrile seizures in young children.
Federal vaccine panels are shifting COVID-19 vaccine recommendations, emphasizing individual-based decision-making for most adults and younger individuals, while prioritizing those 65 and older or with underlying health conditions.
Despite internal divisions and concerns about access barriers, committees ultimately voted against requiring prescriptions for COVID-19 vaccines, aiming to maintain broad access while promoting thorough informed consent processes.
The vaccine policy landscape is highly politicized under Health and Human Services Secretary Robert F. Kennedy Jr., whose reconstituted panel explicitly does not recommend COVID-19 vaccines for all US residents.
Analysis
Center-leaning sources frame this story by emphasizing the politicization of vaccine policy under Robert F. Kennedy Jr.'s leadership and the resulting "chaotic" and "turbulent" changes to COVID-19 vaccine access. They highlight concerns from medical experts about the "erosion of integrity" in the advisory process, selective data use, and the potential for reduced vaccine availability, portraying the new panel's actions as problematic and disruptive.
Articles (48)
Center (22)
FAQ
The panels postponed the vote due to ongoing debate and skepticism regarding the necessity of hepatitis B vaccination at birth, particularly for newborns whose mothers test negative.
The advisory panels recommended against the combined MMRV vaccine for young children due to a slightly increased risk of febrile seizures; instead, they suggest separate MMR and varicella shots.
The federal vaccine panels have shifted COVID-19 vaccine recommendations to emphasize individual-based decision-making for most adults and younger individuals, prioritizing vaccination mainly for those 65 and older or with underlying health conditions.
They voted against requiring prescriptions to maintain broad access to COVID-19 vaccines while promoting thorough informed consent processes, despite internal divisions and concerns about access barriers.
Under Secretary Robert F. Kennedy Jr., the reconstituted vaccine panel explicitly does not recommend COVID-19 vaccines for all US residents, contributing to a highly politicized vaccine policy landscape.
History
This story does not have any previous versions.