Global Obesity Crisis Escalates, WHO Endorses GLP-1 Drugs for Management
The global obesity crisis is projected to affect two billion people by 2030, incurring trillions in costs and millions of deaths, prompting WHO to recommend GLP-1 drugs.
Overview
The global obesity crisis currently impacts over one billion people, with projections indicating a rise to two billion individuals by 2030 if no significant action is taken.
Obesity was linked to 3.7 million deaths worldwide in 2024, primarily due to associated conditions like heart disease and diabetes, highlighting its severe health consequences.
The economic burden of the escalating obesity crisis is substantial, with costs projected to reach an alarming $3 trillion globally by the year 2030.
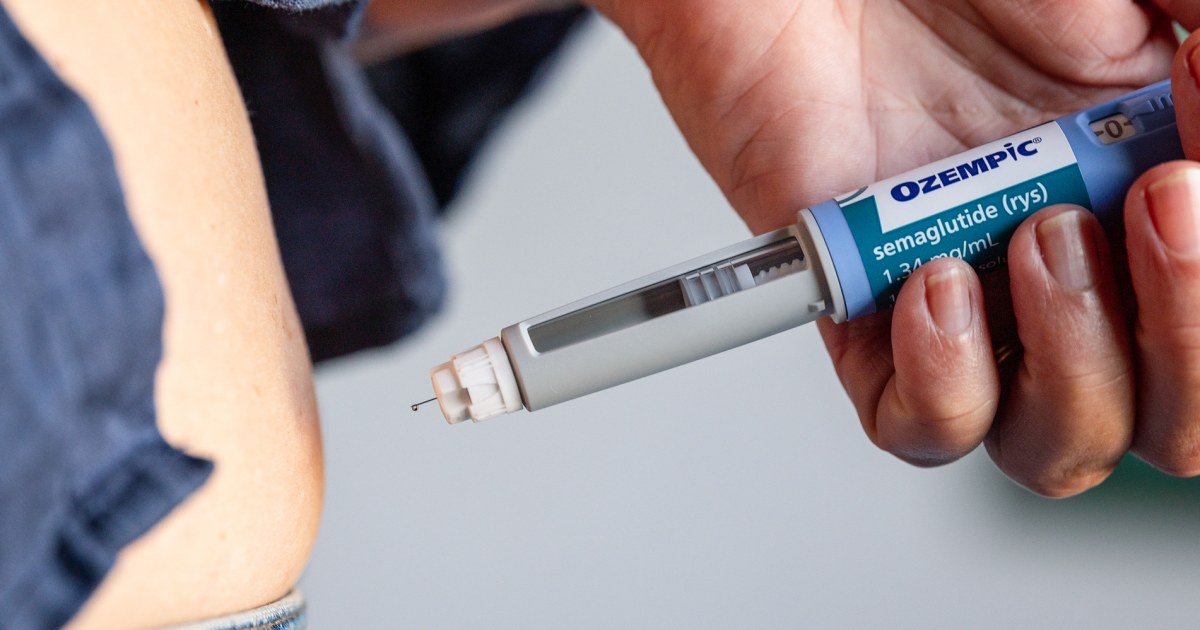
The World Health Organization (WHO) has officially recommended GLP-1 drugs as a new and effective tool for managing obesity in adults.
GLP-1 drugs function by mimicking a natural hormone, effectively slowing digestion, curbing appetite, and increasing feelings of fullness to aid in weight management.
Analysis
Center-leaning sources cover this story neutrally, primarily reporting on the World Health Organization's (WHO) new guidance and warnings regarding obesity medications. They present the WHO's call for equitable access, its reclassification of obesity as a chronic disease, and the challenges of high costs and limited production capacity. The coverage avoids loaded language and provides balanced information on the drugs' efficacy and potential downsides.
Sources (5)
FAQ
GLP-1 drugs mimic a natural hormone that slows digestion, reduces appetite, and increases feelings of fullness, aiding in weight management for adults with obesity.
Common side effects include nausea, vomiting, diarrhea, constipation, fatigue, dizziness, and injection site reactions. Some less common but serious effects can involve gallbladder issues and a potential risk of thyroid tumors.
By 2030, obesity is projected to affect two billion people globally, causing trillions in economic costs and contributing to millions of deaths mainly due to related conditions like heart disease and diabetes.
Yes, unapproved or compounded GLP-1 drugs may pose safety risks such as dosing errors leading to serious adverse effects, including nausea, vomiting, diarrhea, and abdominal pain, sometimes requiring hospitalization.
Gastrointestinal side effects like nausea, vomiting, diarrhea, and constipation are common usually during treatment initiation. Clinical guidelines recommend gradual dose escalation and symptom management strategies to help patients continue therapy safely.
History
This story does not have any previous versions.